This story was originally shared by Emma Nott, Secretary Hope for Hypothalamic Hamartomas, Secretary ePAG ERN EpiCARE 15 September 2020
It is estimated that 1 in around 200,000 people is born with a hypothalamic hamartoma (HH), an epileptogenic lesion attached to the hypothalamus[1]. However, HH syndrome is so rare, its hallmark seizure-type so unusual, and its co-morbidities so often attributed to other developmental conditions such as autism, ADHD and oppositional defiance disorder, that it would be no great shock to discover a much higher prevalence than received wisdom currently suggests.
Rare Epilepsy Syndromes
As with many rare epilepsy syndromes, diagnosis is not straightforward, and for many sufferers it comes much too late. In fact, a significant proportion of those with this paediatric epilepsy syndrome are not accurately diagnosed until adolescence or even adulthood – sometimes after devastating and wrong surgical intervention. One such person is Carrie Fulcher, President of the UK Chapter of Hope for HH. Carrie was misdiagnosed throughout her childhood, adolescence and beyond, into adulthood and motherhood. A succession of radiologists did not detect her HH and told her that her MRIs were normal. Because her seizure activity was displaying itself in the temporal lobe, she was eventually diagnosed as having right temporal lobe epilepsy, which led to a wholly unnecessary temporal lobectomy in 2013. After further deterioration, a more senior radiologist reviewed the MRI and at that point HH was detected. The wholly erroneous and irreversible surgery has affected Carrie cognitively, and caused lasting and debilitating anxiety and depression. Unsurprisingly, Carrie is determined that this should not happen to anyone else.
Hypothalamic Hamartoma (HH)
HH can cause many types of seizures and other symptoms. However, its hallmark seizure type is gelastic seizures – sudden episodes of uncontrolled, often mirthless, laughter[2]. In infancy gelastic seizures can be mistaken for reflux or colic. Infants with HH may miss critical developmental milestones in speech, crawling, walking and cognition. Diagnosing the initial seizures can be hard since symptoms are usually missed or not considered seizures at first. Paediatricians may dismiss parental reports of what is in fact seizure activity as rather describing developmental or autistic-style behaviours. If an EEG is ordered, the results can be misleading, often appearing normal or showing minor changes or non-specific abnormal findings in children.[3] This can lead to a clinician mistakenly ruling out seizures.
MRI and other diagnostic tools
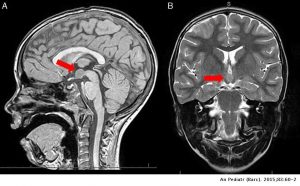
HH can be definitively diagnosed through MRI, performed to a specific protocol[4]. While a properly performed and correctly read MRI scan should bring the diagnostic odyssey to an end, unfortunately for many sufferers the journey continues. HHs are categorised by experts according to size and the nature of their attachment within the hypothalamus; some are small, some are ‘giant’[5]. A giant HH is difficult to miss on an MRI scan; not so the smaller lesions. Even experienced neuroradiologists struggle to identify smaller HHs, perhaps because they are more accustomed to looking for anatomical lesions in the cortex (the more typical location of seizure onset) than in subcortical regions. A significant proportion of our patient community has been advised by the first instance neurological team that their MRI has ruled out HH, only for a specialist HH or epilepsy centre to diagnose HH upon second opinion.
Related endocrine issues
In about a third of cases the HH creates significant endocrine issues; in particular, it can cause precocious puberty[6]. In such cases is it often the precocious puberty that provides the clue that leads to accurate diagnosis, notwithstanding a longer-standing history of seizures in the patient. One parent of a two-year old girl who showed symptoms of menstruation, has described her situation as ‘fortunate.’ She believes that without the precocious puberty diagnosis, which then led to discovery of an HH, her daughter – said by her paediatrician to be autistic – would have continued to have been misdiagnosed and continued to have deteriorated cognitively and behaviourally. Accurate, early diagnosis is critical, as timely surgical intervention can control or significantly reduce the seizures and arrest cognitive and behavioural decline[7].
We do not yet know the cause in all cases of HH but believe genetic factors contribute to many of them. A small percentage of children with HH have Pallister Hall Syndrome, an inherited genetic condition where abnormalities of the GLI3 gene affect changes in the way other parts of the body develop: that may include extra fingers or toes, changes in hormone function, bifid epiglottis, imperforate anus and kidney abnormalities[8]. However, the majority of HH cases are not inherited from a parent, and instead arise randomly in the child. In such cases patients and families are regularly told by their clinicians that since the HH is sporadic and not apparently syndromic there is no reason for genetic investigation. A team of scientists at the University of Melbourne is currently examining tissue from ‘sporadic’ HHs removed by surgery. In about one third of HHs examined to date, there is an abnormality in the GLI3 gene, or a related gene with a similar function during development. Abnormalities in other types of genes may cause HH and there is active research underway to find these.[9]
The role of genetic couselling
Genetic counselling is recommended for all individuals with Pallister-Hall syndrome where the GLI3 gene abnormality has been transmitted from a parent to their child because the same abnormality can be passed on to other children.[10] It is not currently recommended for non-syndromic cases however that may change depending on further research. From the patient point of view, the uncertainty over the causes of HH – and the current reluctance of the clinician to refer for genetic counselling in the absence of signs of Pallister-Hall Syndrome – is frustrating. A definitive answer regarding the cause of a child’s HH may help inform the parental approach to treatment, and also to family-planning – for both the parents and, in future, for the child and any siblings.
On occasion, genetic testing might form part of the diagnostic odyssey: in one case, neurologists and radiologists at different epilepsy centres disagreed whether HH was indicated on the patient’s MRI. The diagnosing team pointed to the clinical manifestation of repetitive, short bursts of unexpected, uncontrolled laughter – gelastic seizures – as supporting the presence of a small HH visible on the MRI. The other team countered that the MRI showed no more than a slight thickening of the third ventricle wall, and that the laughing episodes were equally consistent with behavioural manifestations of autism. EEG and VEEG were inconclusive. Having to choose between the two teams – both eminent, both certain – the parents were ultimately assisted by a genetic test revealing a previously unreported genetic mutation on the GLI3 gene. Consequent Gamma Knife surgery significantly ameliorated the behavioural and cognitive dysfunction caused by the HH. This case concerned my son, who was seven years old at the time. He had been consistently misdiagnosed until that age as having childhood autism and ADHD.
Hope for Hypothalamic Hamartoma
More than a decade later – notwithstanding the efforts of Hope for HH and the expert doctors who guide us – parents come to us with similar stories. Even when HH – or seizure activity – is suspected, there appears to be a reluctance by the treating clinician or hospital to refer the patient on to a specialist epilepsy centre. MRIs are still being performed to a standard protocol, rather than to the protocol advised by paediatric epilepsy experts; MRIs are still being misread. Parents are still being advised that because their child’s EEG is normal, seizures can be ruled out.
This is one of the reasons why we at Hope for HH believe it is so important to have an International HH Awareness month, incorporating the first International HH Awareness Day this 15th September. If doctors do not know what HH or gelastic epilepsy is, then they cannot diagnose it. If they do not refer a patient with suspected seizures to a specialist epilepsy centre, then that patient’s outcome is likely to be sub-optimal. If they misdiagnose the fact or the cause of the seizures, the consequent treatment – or lack of treatment – might be catastrophic.
We are rare – ultra-rare. Sadly, due to the perils of the diagnostic odyssey, we are probably not as rare as we would like to think.
Originally shared by Emma Nott, Secretary Hope for Hypothalamic Hamartomas, Secretary ePAG ERN EpiCARE 15 September 2020
References:
[1] Berkovic, S.F., Arzimanoglou, A., Kuzniecky, R., Harvey, A.S., Palmini, A., Andermann, F., 2003. Hypothalamic Hamartoma and Seizures: A Treatable Epileptic Encephalopathy. Epilepsia 44(7), 969-973: https://pubmed.ncbi.nlm.nih.gov/12823582/ [2] Berkovic, et al 2003; Kerrigan, J.F., Ng, Y.T., Chung, S., Rekate H.L., 2005. The hypothalamic hamartoma: a model of subcortical epileptogenesis and encephalopathy. Semin Pediatr Neurol 12(2):119-131.https://europepmc.org/article/med/16114178 [3] Kerrigan et al, 2005 https://onlinelibrary.wiley.com/doi/full/10.1111/epi.13752 [4] Berkovic et al, 2003 https://www.genome.gov/staff/Leslie-G-Biesecker-MD [5] Delalande O, Fohlen M: Disconnecting surgical treatment of hypothalamic hamartoma in children and adults with refractory epilepsy and proposal of a new classification. Neurol Med Chir (Tokyo) 43:61-68, 2003 https://pubmed.ncbi.nlm.nih.gov/12627881/ [6] Harrison, V.S., Oatman, O., Kerrigan, J.F., 2017 Hypothalamic hamartoma with epilepsy: Review of endocrine comorbidity. Epilepsia 58(Suppl 2):50-59. https://pubmed.ncbi.nlm.nih.gov/28591479/ [7] Berkovic et al 2003; Kerrigan et al 2005 https://onlinelibrary.wiley.com/doi/full/10.1111/epi.13752;https://www.ncbi.nlm.nih.gov/books/NBK1465/
[8] Biesecker, LG., 2000. Pallister-Hall Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews®. Seattle (WA): University of Washington, Seattle. https://www.ncbi.nlm.nih.gov/books/NBK1465/ [9] Hildebrand, M.S., Griffin, N.G., Damiano, J.A., Cops, E.J., Burgess, R., Ozturk, E., Jones, N.C., Leventer, R.J., Freeman, J.L., Harvey, A.S., Sadleir, L.G., Scheffer, I.E., Major, H., Darbro, B.W., Allen, A.S., Goldstein, D.B., Kerrigan, J.F., Berkovic, S.F., Heinzen, E.L., 2016. Mutations of the sonic hedgehog pathway underlie hypothalamic hamartoma with gelastic epilepsy. American Journal of Human Genetics 99(2), 423-429; Fujita, A., Higashijima, T., Shirozu, H., Masuda, H., Sonoda, M., Tohyama, J., Kato, M., Nakashima, M., Tsurusaki, Y., Mitsuhashi, S., Mizuguchi, T., Takata, A., Miyatake, S., Miyake, N., Fukuda, M., Kameyama, S., Saitsu, H., Matsumoto, N., 2019. Pathogenic variants of DYNC2H1, KIAA0556, and PTPN11 associated with hypothalamic hamartoma. Neurology 93(3), e237-e251. https://www.thermh.org.au/research/publications/6966 [10] Biesecker, 2000








Leave a Reply